
- Understand how well all that we have discussed so far works
- Understand what went wrong

|
Objectives: by the end of this you will
|

The layer of prejudices we acquire before we are sixteen" A. Einstein
| Found black lines superimposed on sun's spectrum. |  |
| Heated gases give characteristic wavelengths, and can match dark lines in solar spectrum to bright emission lines: e.g. Sodium |  |
| Hydrogen is simplest | 
|

| Light falls on metal, ejects negative charge, which we now know consists of electrons: can measure energy of electrons by stopping them in an electric field |  |
| J.J. Thomson's discovery of electron and measurement of e/m use velocity separator consisting of both mag. and E.S. fields: | 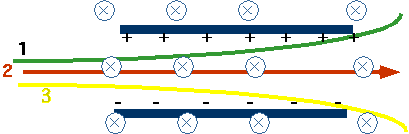 |
Electric Force
FE = qEMagnetic Field into screen so force FM= Bqv |

|
qE = Bqv or v= E/B
| (Thomson): Currant bun model of atom,: electrons imbedded in positively charged material. |  |
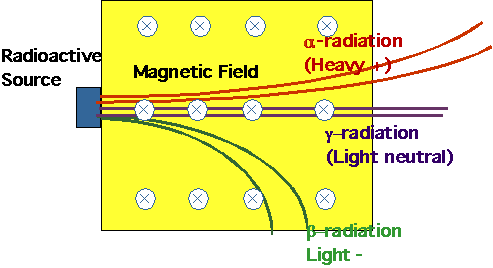
"Something" penetrating given off by certain materials (e.g. uranium
salts). Subsequently saw that
|
|
T(K) = T(oC) + 273
| Note that this assumes that the body is a perfect radiator: a black-body. |  |
| To derive a theory of Black body radiation put E.M. standing waves in cavity. no particular reason to assume that any one wavelength preferred to any other: short waves dominate (since there are more of them) |  |
| this gives Rayleigh-Jeans curve. The "ultra-violet catastrophe": all radiation should be very short λ. |  |
| Wien noticed that experimental curve looked like Maxwell-Boltzmann distribution. for molecules |  |
| Röntgen (1895) |  |
| Very penetrating radiation produced by vacuum tube (requires pot. diff ~ 20 kV), which passes through solids, fogs photographic plates | 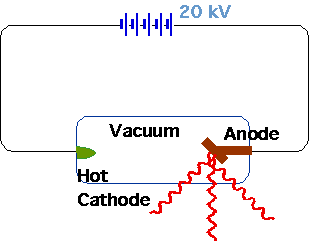 |
 |
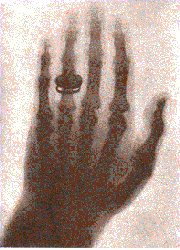 |
After Röntgen, Moseley found that each atom gave characteristic X-rays
E = 10.26(Z-1)² eVfor most energetic X-Ray (Kα)
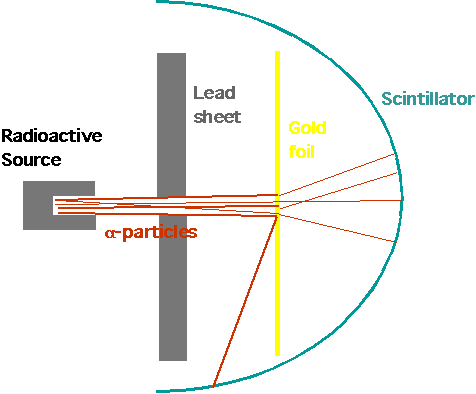
| Rutherford (1908)
Lead block with radium salt: α-particles are produced by radium, collimated by screen to narrow beam, pass through gold foil and are detected by scintillator (produces spark of light when hit by charged particle |
|
| Expect: rather small angle of deflection due to many small scatters | 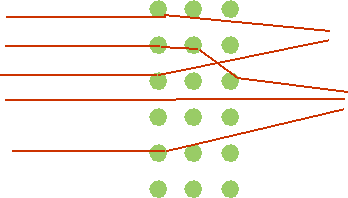
|
Close encounter with electron?
me ~ 1 mα 8000 |
 |
|
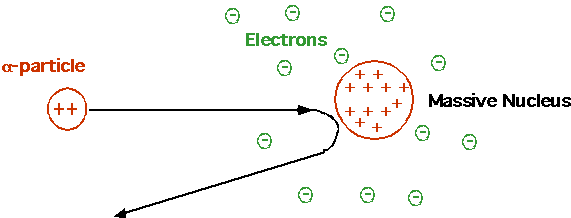
Cannonball bouncing backwards off tissue paper. Rutherford Need small, massive, positive charged nucleus. Electrons irrelevant! |
 |
| Why don't the electrons fall into nucleus? |  |
| Maybe electrons are in orbit but an accelerating charge emits radiation so orbiting electrons should emit radiations and lose energy. |  |