
A wavicle
- Are particles really just solid objects?
- How do we describe a particle as a wave?
- What is Schrodingers equation?
- How accurately can we measure things?
- How do complex atoms arise
- How do spectra arise?
- What are X-rays
 A wavicle |
Objectives: by the end of this you will be able to answer the following
|
| used crystal as diffraction grating: (2-D so pattern is more complicated) | 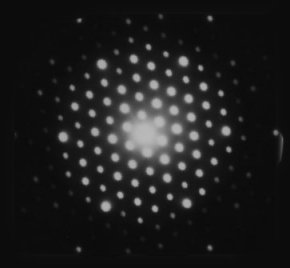 |
| A simpler experiment is now possible: the electron analog of Young's slits. Very low energy electrons pass through slits and hit detector (e.g. photo plate) and give 2-slit interference pattern | 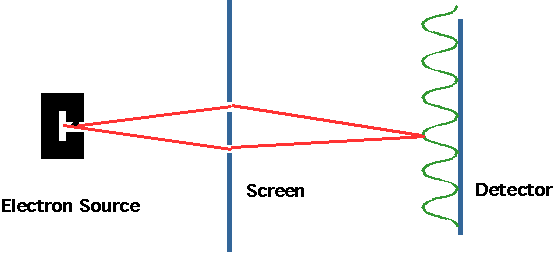 |
| A dramatic recent example uses a buckyball C60 American Journal of Physics, Vol. 71, No. 4, p319, April 2003, Nairz, Arndt, and Zeilinger |
 |
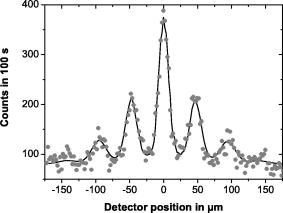
| Apparatus uses a diffraction grating:velocity v = 117 ms-1 |  |
Circles are the experimental data. Line represent the model
|
 |
| Newton:
\color{red}{
E = \frac{1}{2}mv^2 }
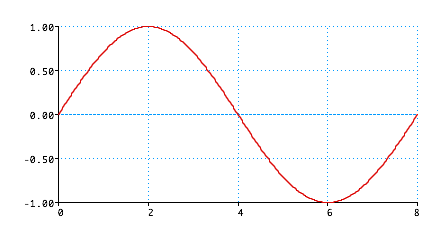
De Broglie:
Standing wave
\color{red}{
\lambda = \frac{h}{p}}
Wave (like guitar string)
\color{red}{
\lambda = \frac{{2L}}{n}}
|
|

Heisenberg 1927
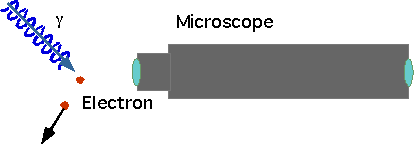
If an electron is a wave, how can we define its position?
Uncertainty in position δx = Lbut there is also an uncertainty in momentum δp~2p~2h/λ=h/L |
 |
δxδp = L h/L = h
δx >λSo decrease wavelength to get position better, but photon carries momentum p=h/λand some of it gets transferred |
|
δx δp >λ(h/λ) >h
This is a fundamental limitation on human knowledge: can always do worse but cannot do better!
δE δt > h
De Broglie suggested that allowed orbits have an integral number of waves fitted
R = nh
2πp
in 2πR = nλ= nh/p |
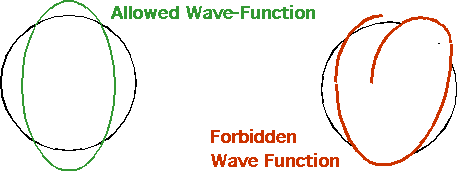 |
pR = mvR = nh/2π = nħ = L
En = -13.6 eV
n²
En = -13.6Z² eV
n²
| Note that the energy depends only on n. | 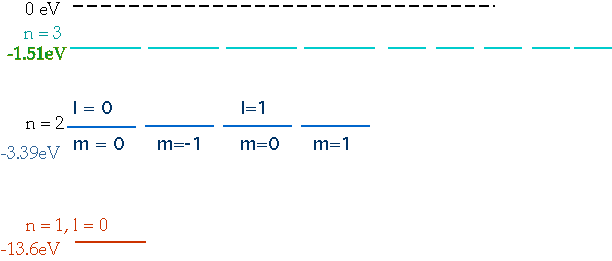 |
| must have number of electrons = Z = charge on nucleus, and fill lowest energy levels first. | 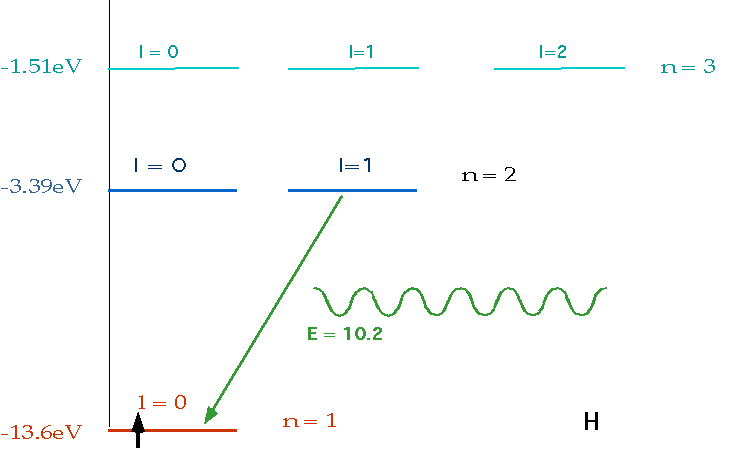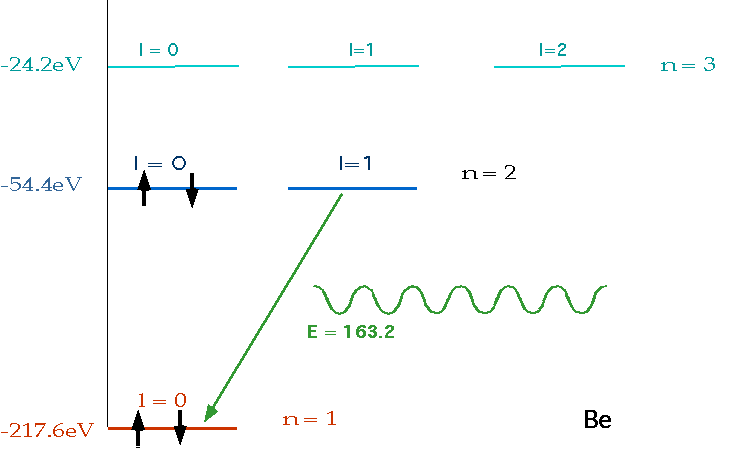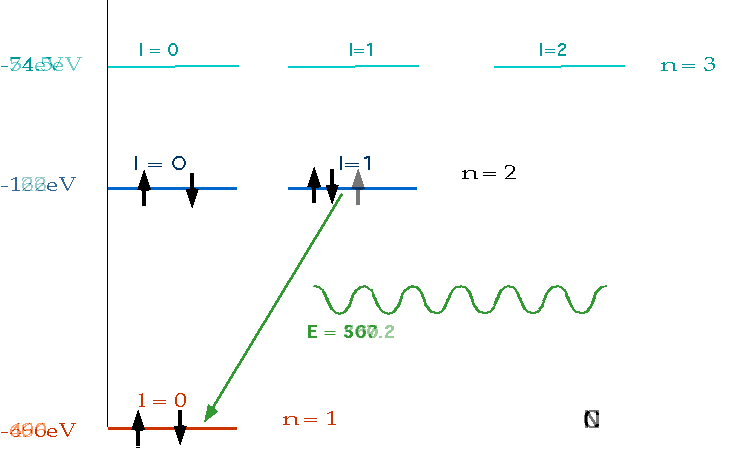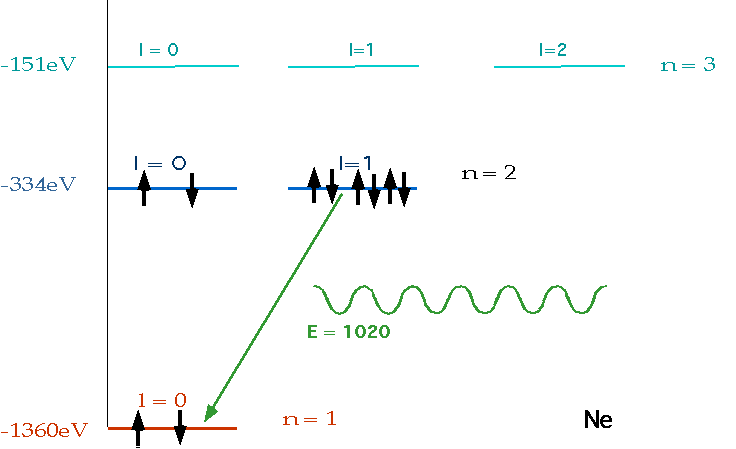 |
| If electron makes transition from one level to another, we will get emission line of definite energy | 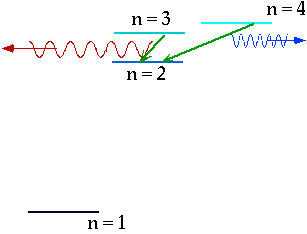
|
| However, if we have photons of all energies, one may have exactly the energy to raise the energy of an electron | 
|
| With care, can see both absorption and emission at the same time. | 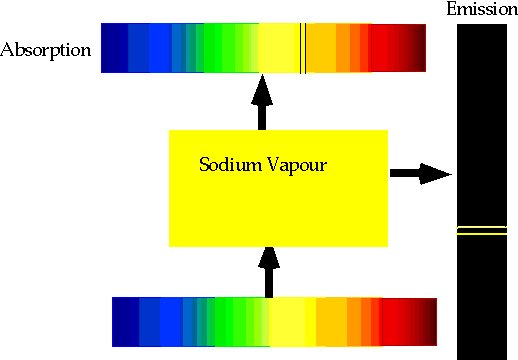 |
| Electron accelerated | 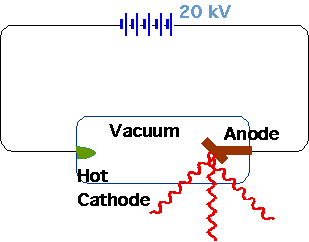 |
| Electron collides with atom, knocks out electron in lowest energy level, leaves vacancy for electron in higher level to fall into.e.g. for e.g Chromium: (Z = 24) | 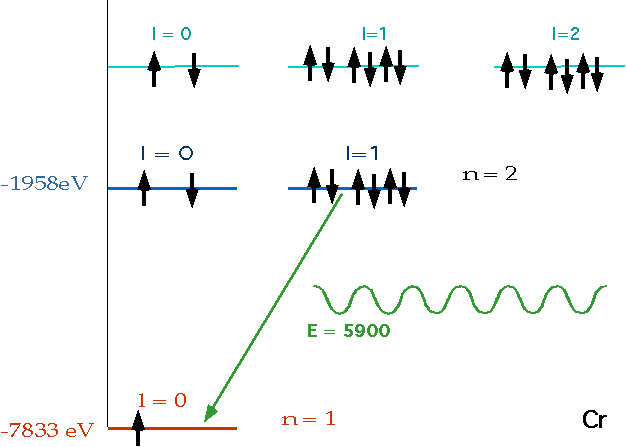 |
| SEM (Scanning Electron Microscope | 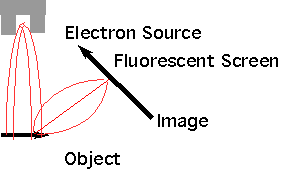 |
| e.g ant with microchip (from the Science Museum, London) | 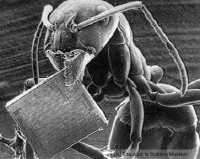 |
| TEM (Transmission Electron Microscope) | 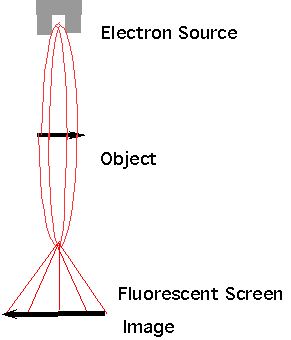 |
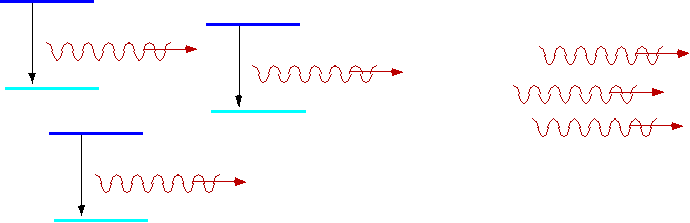
| However, if we have an atom in an excited state, a second photon γ will cause it to decay, and then the new photon will be added to the first. |
 |
| Hence find an atom with 3 levels, E₁,E₂,E₃, one of which is metastable. Get the atom into the 3rd state (by collision or excitation). Then (e.g) it drops into E₂ by spontaneous emission and then remains there until a second photon hits it | 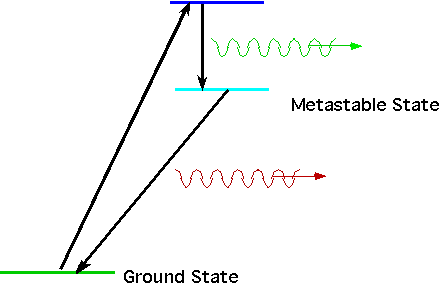 |