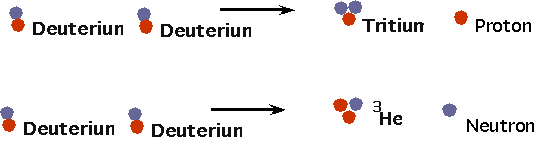
|
A wavicle |
| used crystal as diffraction grating: (2-D so pattern is more complicated) | 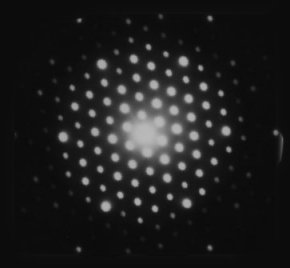 |
| A simpler experiment is now possible: the electron analog of Young's slits. Very low energy electrons pass through slits and hit detector (e.g. photo plate) and give 2-slit interference pattern | 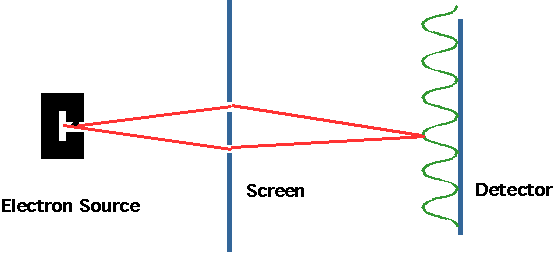 |
| A dramatic recent example uses a buckyball C60 American Journal of Physics, Vol. 71, No. 4, p319, April 2003, Nairz, Arndt, and Zeilinger |
 |
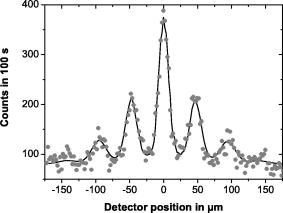
| Apparatus uses a diffraction grating:velocity v = 117 ms-1 |  |
| Circles are the experimental data. Line represent the model
A buckyball C60 has a mass of 60*12*1.67x10-27 = 1.2x10-24 kg. The speed is 117 ms-1. What is λ for these parameters? |
 |
| Newton:
\color{red}{
E = \frac{1}{2}mv^2 }
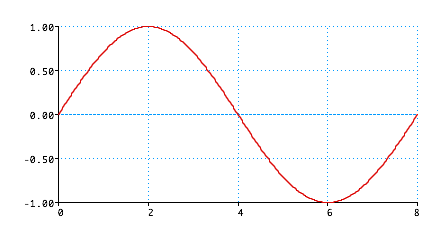
De Broglie:
Standing wave
\color{red}{
\lambda = \frac{h}{p}}
Wave (like guitar string)
\color{red}{
\lambda = \frac{{2L}}{n}}
|
|

Model for H. atom must explain
n = 1 m = 2, 3, 4... n = 2 m = 3, 4, 5... n = 3 m = 4, 5, 6...
|
\color{red}{
F = \frac{{Gm_1 m_2 }}{{r^2 }} \Rightarrow \frac{{kq_1 q_2 }}{{r^2 }} = \frac{{mv^2 }}{{r^2 }}}
|
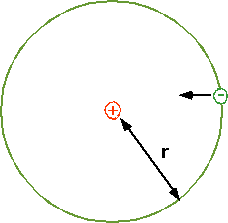 |
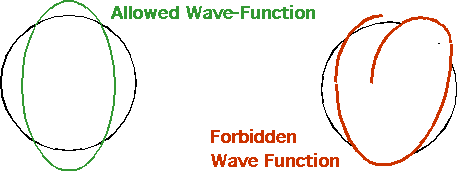
| De Broglie suggested that allowed orbits have an integral number of waves fitted into one orbit
\color{red}{
\lambda = \frac{{2\pi r}}{n} = \frac{h}{p}}
|
 |
because!
| These levels have energies
\color{red}{
E_n = -\frac{{13.6}}{{n^2 }}eV}
|
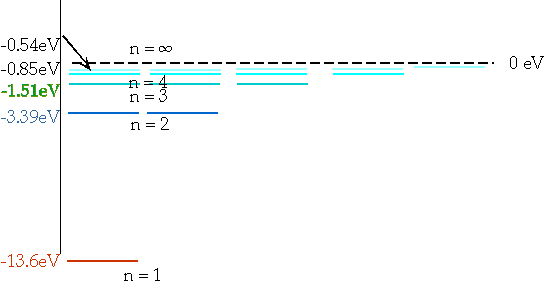 |
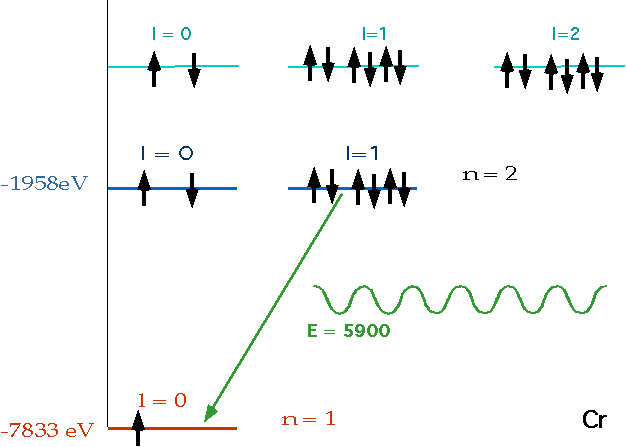
En = = - 13.6 eV
n²
e.g. n = 3 ⇒ n = 2δE = E3 - E₂ = 1.9 eV |
 |
|
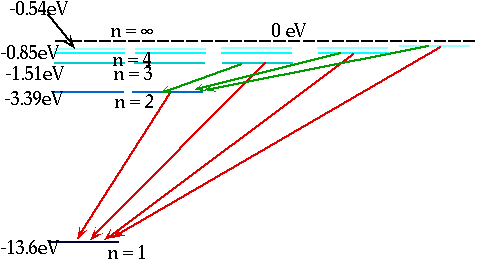 |
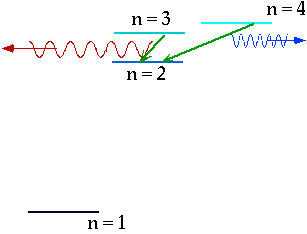
| If electron makes transition from one level to another, we will get emission line of definite energy | 
|
| However, if we have photons of all energies, one may have exactly the energy to raise the energy of an electron | 
|
| With care, can see both absorption and emission at the same time. | 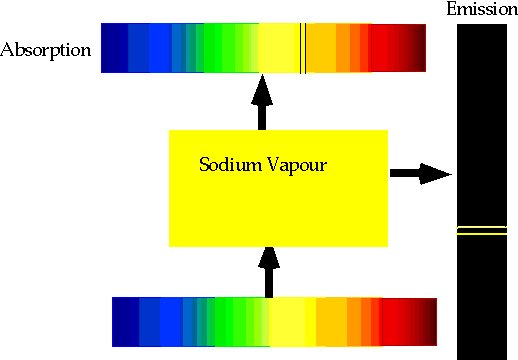 |
Are these energy levels so weird?
Heisenberg 1927
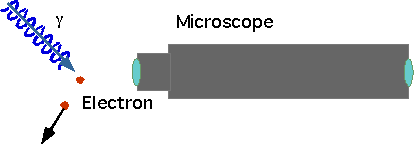
If an electron is a wave, how can we define its position?
Uncertainty in position δx = Lbut there is also an uncertainty in momentum δp~2p~2h/λ=h/L |
 |
δxδp = L h/L = h
δx >λSo decrease wavelength to get position better, but photon carries momentum p=h/λand some of it gets transferred |
|
δx δp >λ(h/λ) >h
This is a fundamental limitation on human knowledge: can always do worse but cannot do better!
δE δt > h
| Mendeleev had found the periodic table in ~ 1850. Very complex pattern: e.g Very reactive acid-forming element (e.g. Cl = Chlorine) is always followed by an inert gas (e.g. Ar = Argon) is followed by a reactive alkaline metal (e.g. K = Potassium) | 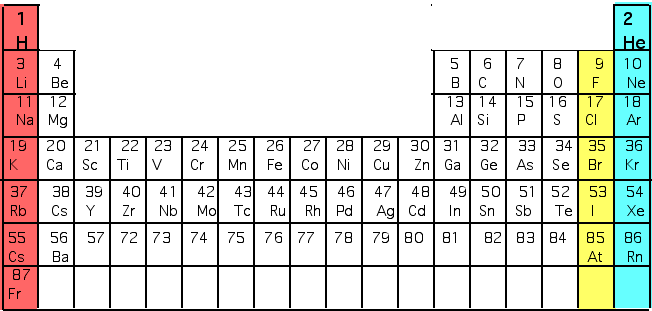 |
En = -13.6Z² eV
n²
| Note that the energy depends only on n. | 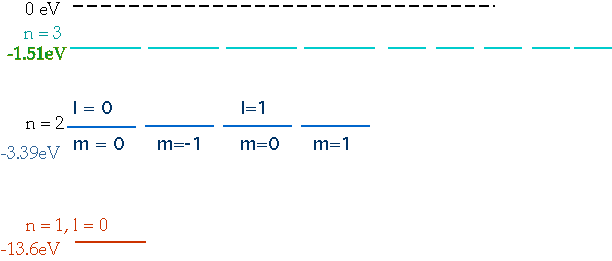 |
| must have number of electrons = Z = charge on nucleus, and fill lowest energy levels first. | 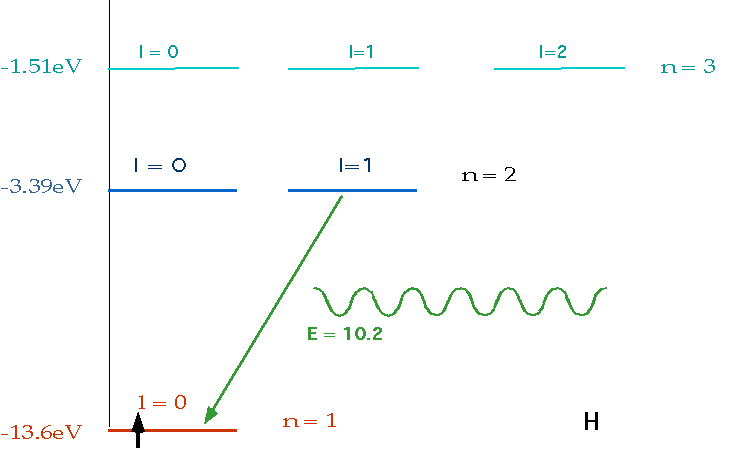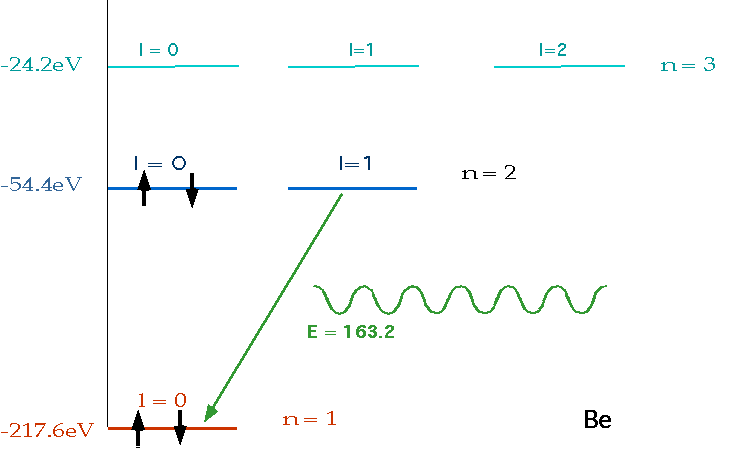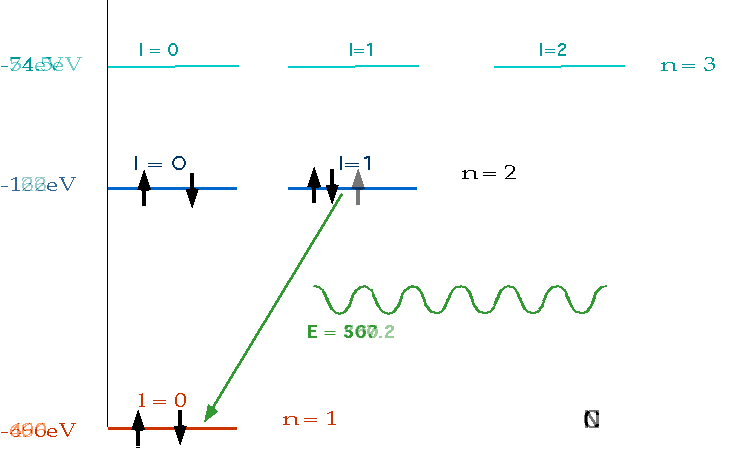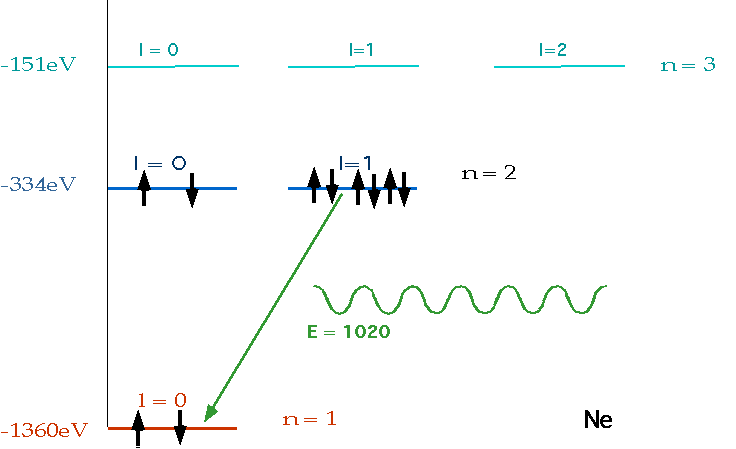 |
| Electron accelerated | 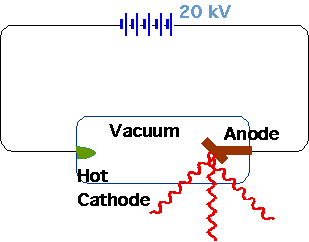 |
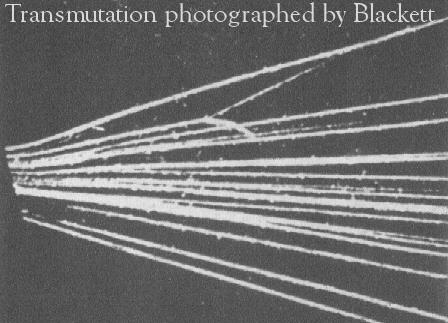
discovered as nucleus of H by Rutherford and Blackett (1921) in
α + N ⇒ O + p
|
 |
Atomic Number Z = charge on nucleus = Nprotons |
This defines chemistry |
Mass number A = Nprotons + Nneutrons |
Isotopes: nuclei with different A but same Z. |
| Usual notation is AZXN but often just write a nucleus as (e.g) 35S, since the name implies Z. In this notation: | 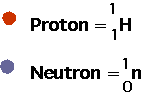 |
E = mc²Usually easier to quote elementary particle masses in terms of energy, measured in eV
= mc² = 9.1x10-31x(3x108)²/1.6x10-19 = 511 keV = .511 MeV
n ⇒ p + γ |
is forbidden: algebraic sum of charges at end of reaction = sum at start |
R ~ R₀ A1/3 where R₀~ 1.4 fm
δxdp ~ h
| Nuclei build up in much the same way as atoms in the periodic table: force are much more complicated, so cannot really solve for the energy. Light nuclei: | 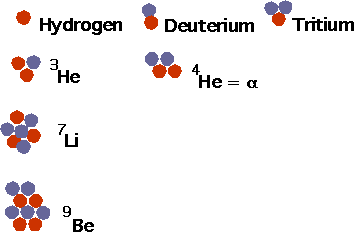 |
| Note that there are no stable elements of A = 5 or A = 8: if you make 8Be it will decay instantly into 2 4He. | 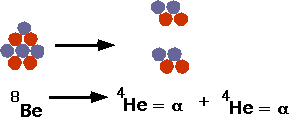 |
| Many nuclei can be created but only a few are stable: this shows nuclei up to oxygen. | 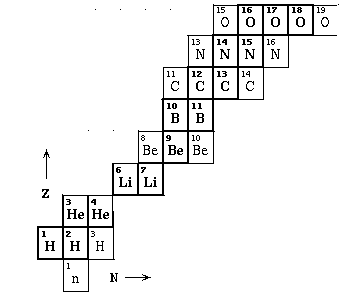 |
| Whole pattern shows N ~ Z for light, N > Z for heavy. |  |
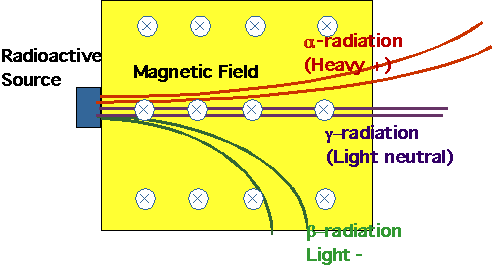
| Becquerel:
Radio-activity and nuclear decays: have already seen 3 varieties. |
 |
Simplest conceptually is γ
decay: just as atoms have energy levels, so do nuclei. . However, they are more
complicated:
|
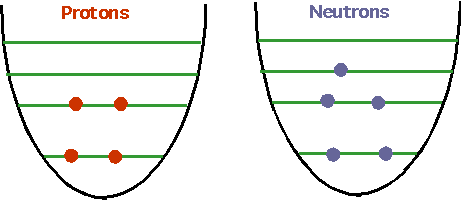 |
| One of the protons (or neutrons) can make a transition if there is a gap.Energy is much higher than in atoms: ~ 10 MeV. |
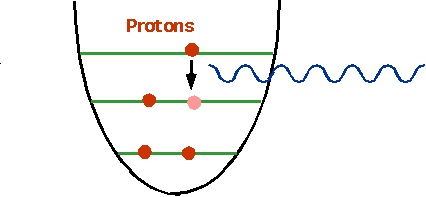 |
n ⇒ p + e-
| This led to a huge problem:the electron came out with varying energy. | 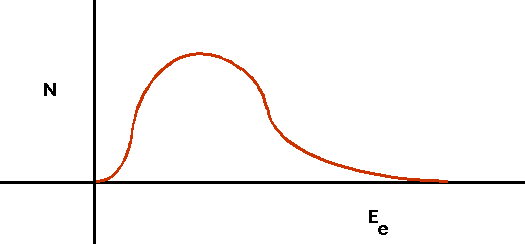 |
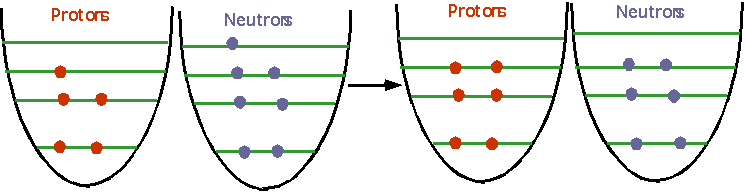
n ⇒ p + e- + ν
This can also happen in a nucleus if the energies are favorable: e.g.
could
have 12B ⇒ 12C + e- + ν |
 |
| When the object dies, no more 14C is absorbed, and what is already there decays back to 14N, with a half-life of 5700 years. | 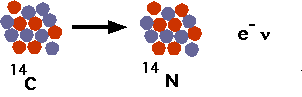 |
|
 |
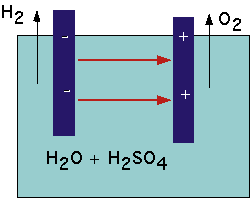
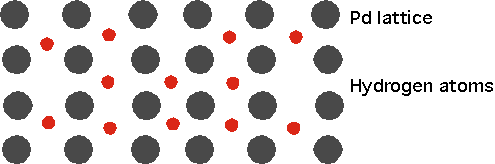 for several days.
for several days.
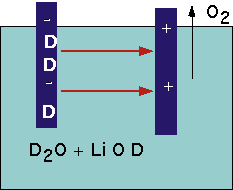
 Is not allowed in free space: instead we should get
Is not allowed in free space: instead we should get