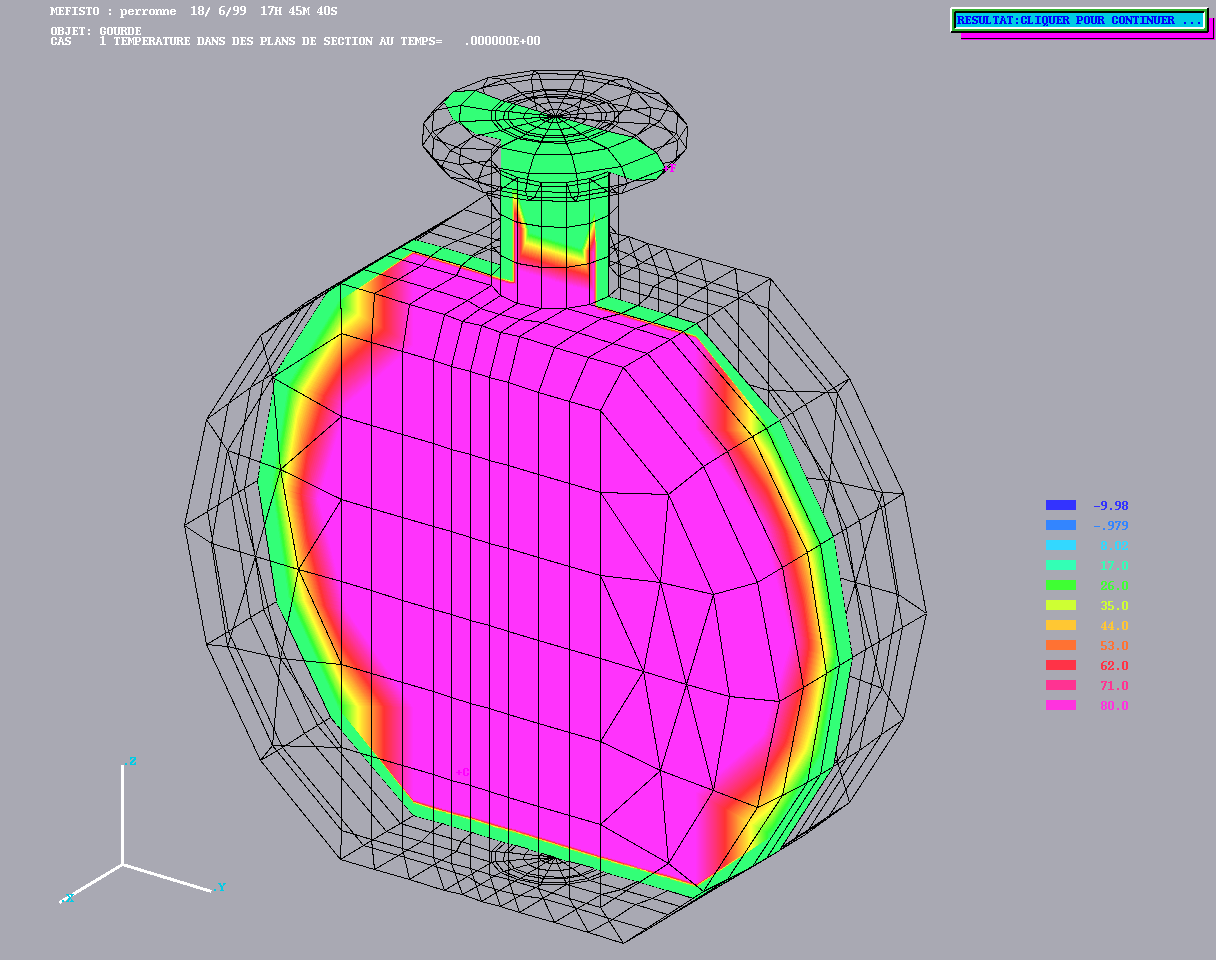
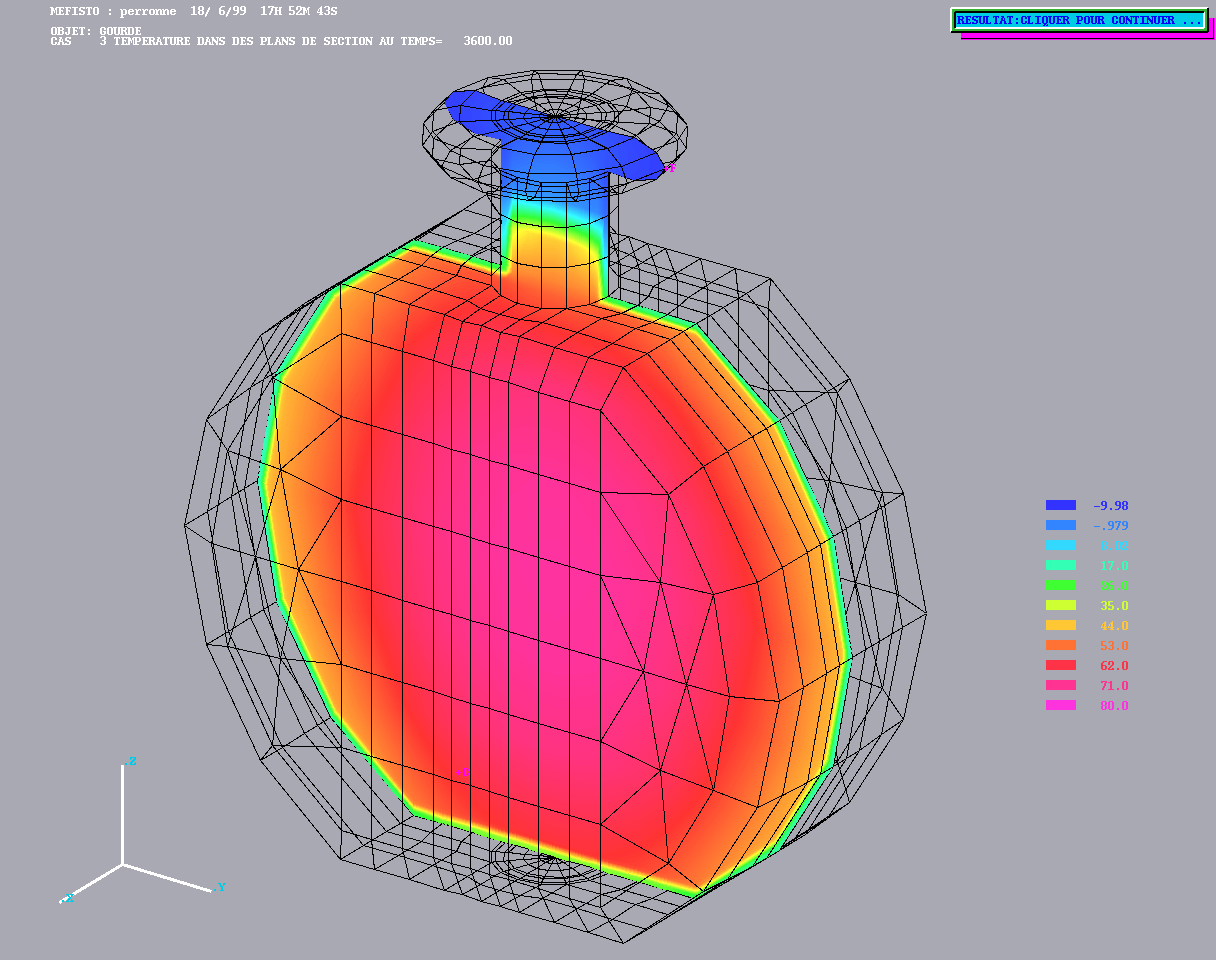
Cooling of a coffee container from Alain Perronnet http://www.ann.jussieu.fr/~perronnet
Macroscopic Heat
- Heat capacity
- Latent Heat
- Thermal Expansion
- Heat transfer
  Cooling of a coffee container from Alain Perronnet http://www.ann.jussieu.fr/~perronnet |
Macroscopic Heat
|
| Experimentally, if we supply heat to a solid, we see is a curve like | 
|
Specific heat is heat capacity for 1 kg.
Heat required to change phase
Note that at certain temperatures
the addition of heat doesn't change the temp, but does change the material from
|
 |
| Most materials expand when heated: Arises because the force-law between atoms is not symmetrical. Hot (i.e. energetic)
atoms will be slightly further apart on average than cold ones.
\color{red}{
\frac{{\delta L}}{L} = \alpha \delta T}
|

|
| Water has a bizarre behaviour,being most dense at 4°C. Note water is also peculiar in that it expands when frozen ⇒ severe difficulties for biological systems. | 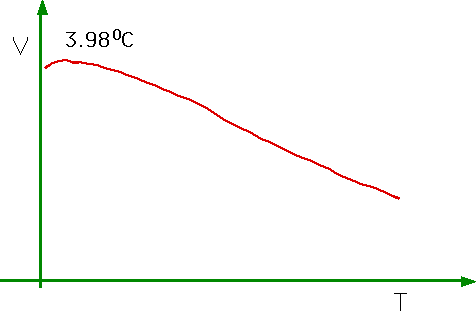 |
Three basic mechanisms
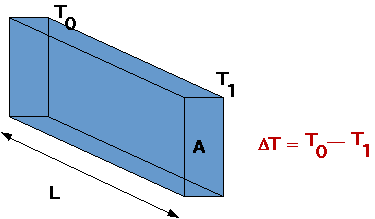
| Energetic atoms in one place share energy with cooler neighbours
Note that it takes time for energy to be transferred. |
 |
k ∼ .08 for fibreglass.
T(K) = T(oC) + 273
Total Energy \color{red}{U = \sigma T^4 ,\sigma = 5.67 \times 10^{ - 8} Jm^{ - 2} K^{ - 4} }
black → red → orange → yellow → white
![]()
| Putting these together gives black-body curve: | 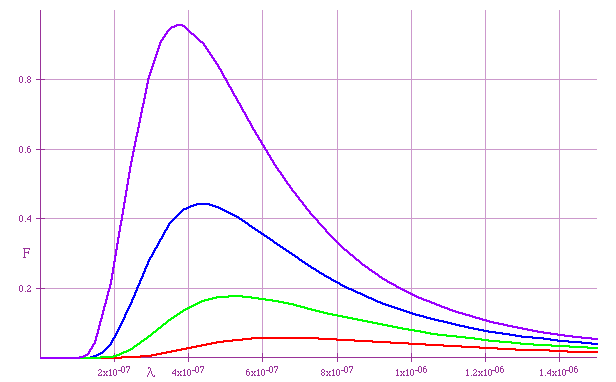 |
| The Earth is not a perfect B-B absorber/radiator: the atmosphere is opaque to some wavelengths, in particular I-R is strongly absorbed around 1-10µM. | 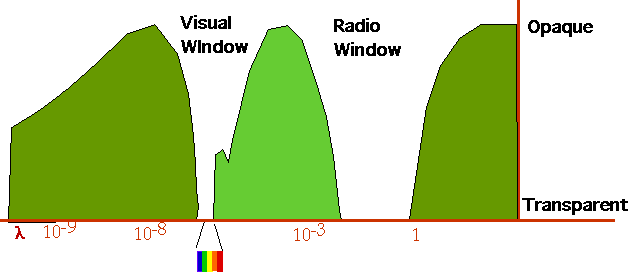 |
| Note that hot fluids have lower density, hence will rise in a gravitational
field (Archimedes). Hence steady circulation set up, which removes heat from centre |
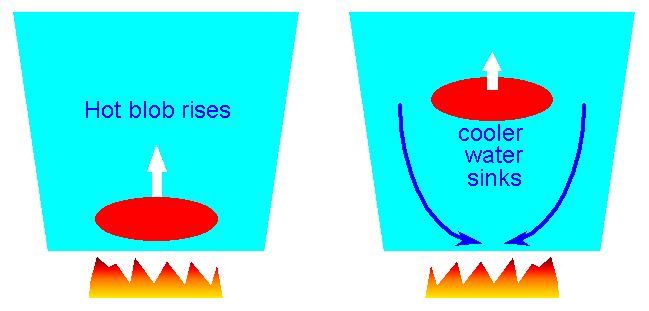 RIchard Pogge Ohio State |
| In reality, almost every process invloves all of the effects: e.g. waiting for your coffee to cool | 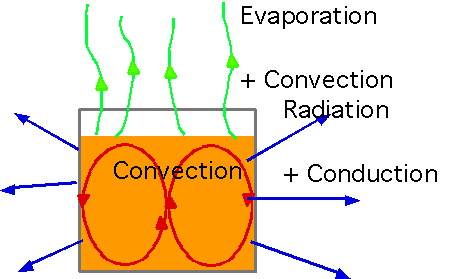 |