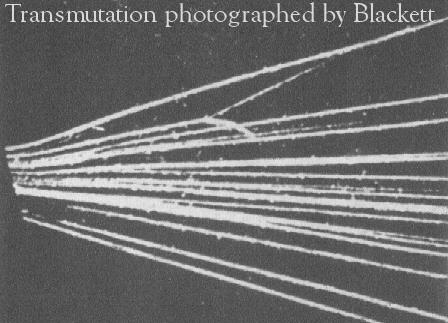
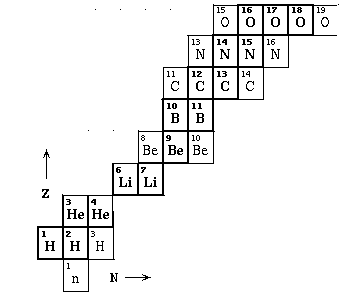
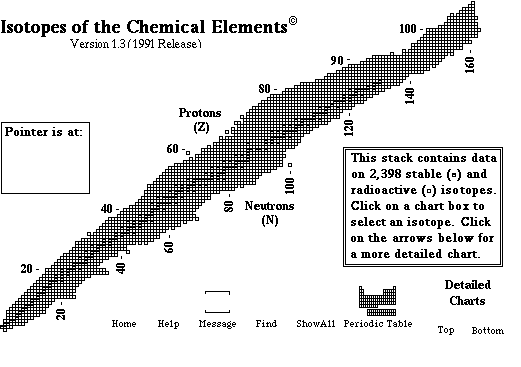
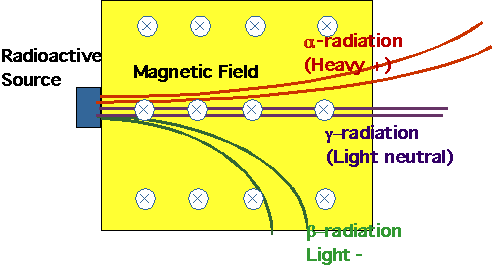
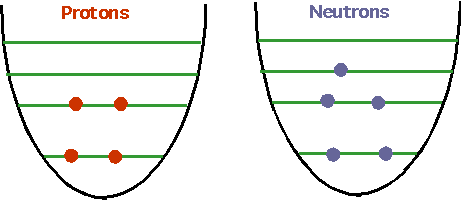
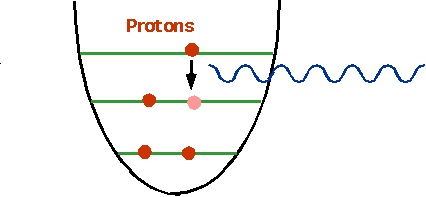
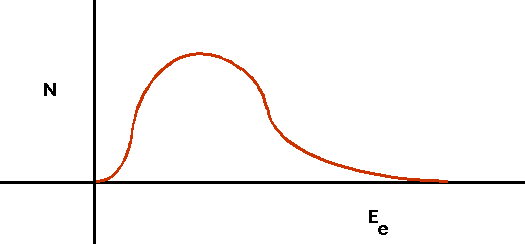
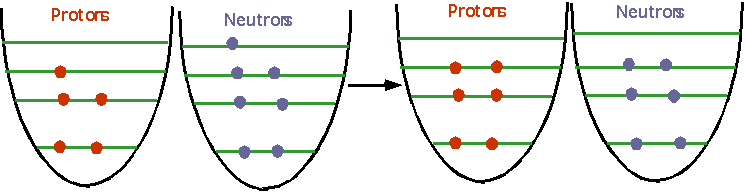
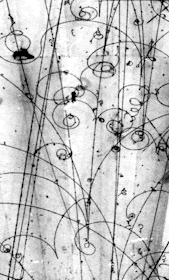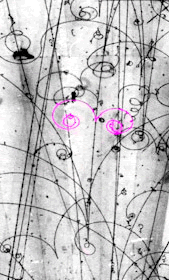
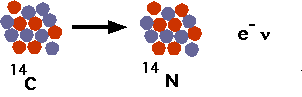
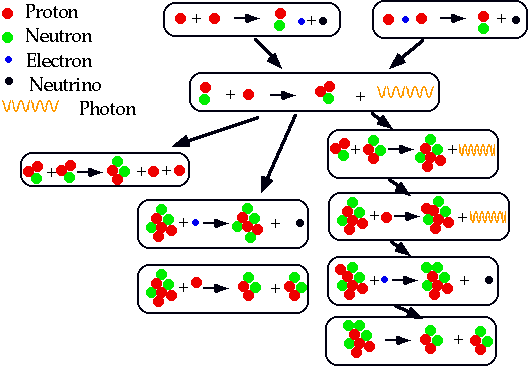
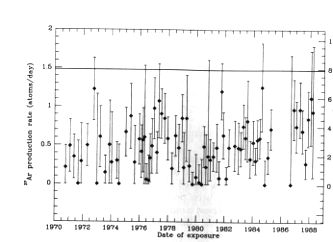
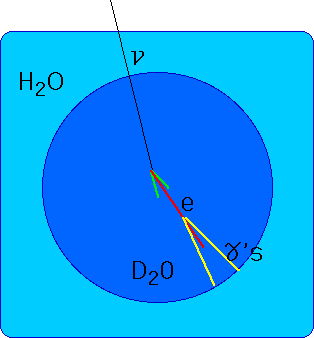
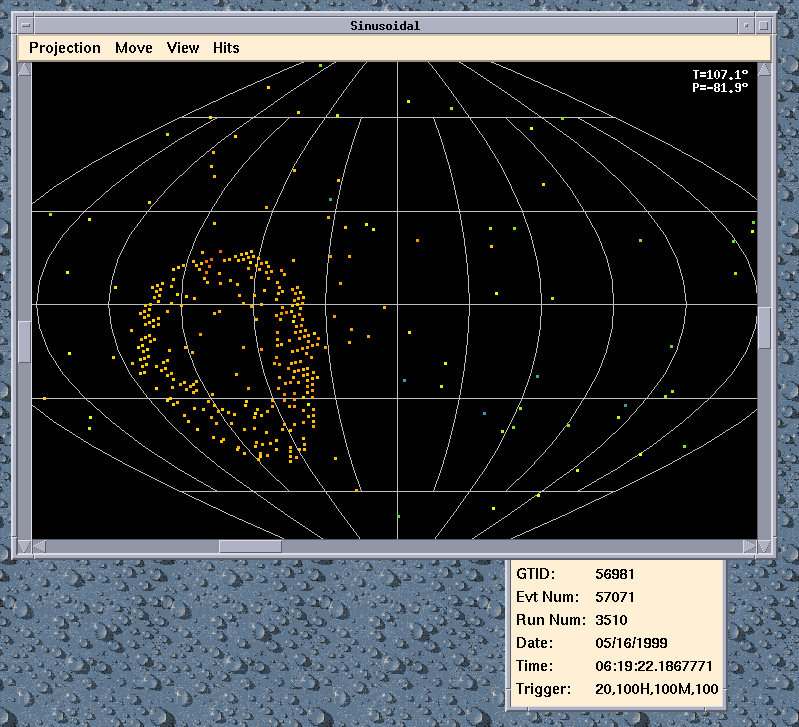
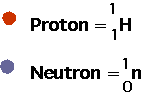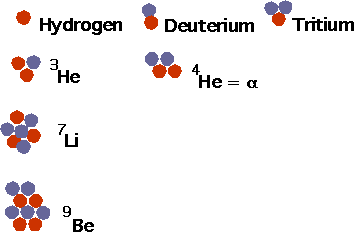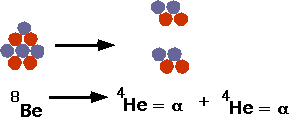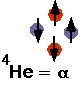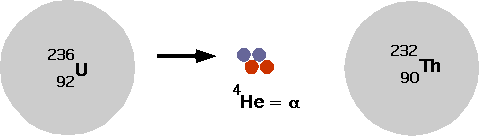- understand how nuclei are made up of protons and neutrons
- understand what isotopes are
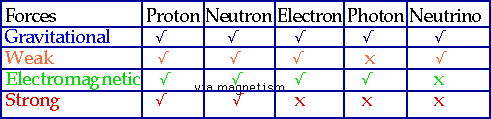
- use conservation laws to explain what can happen in nuclear processes
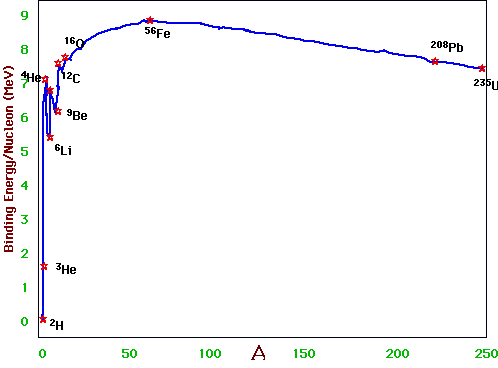
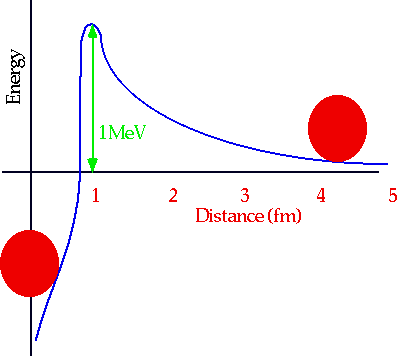
- understand fusion and fission
- understand radioactive decays
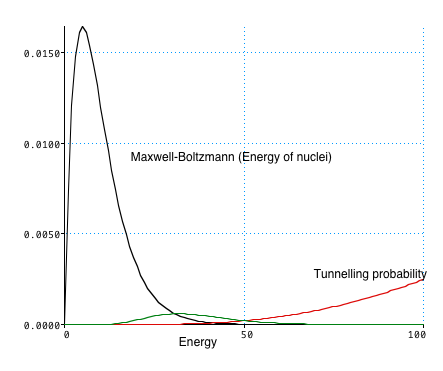
- understand how the sun works
- understand why neutrinos matter